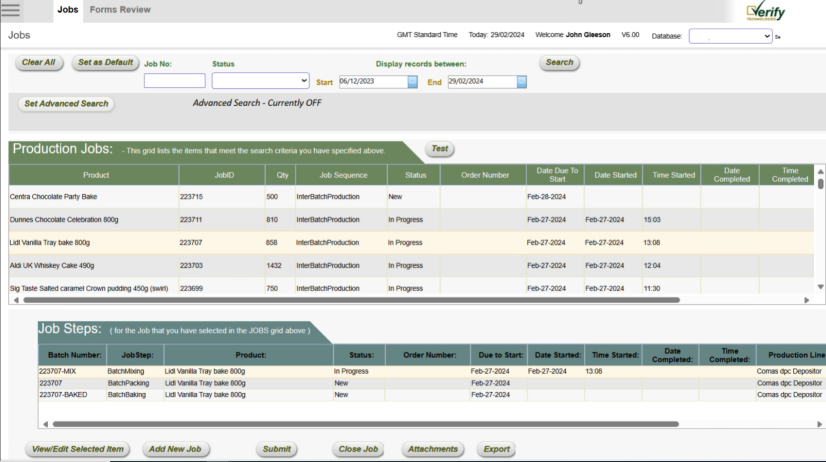
In the critical fields of medical devices and pharmaceuticals, precision, compliance, and efficiency are paramount. Our Electronic Batch Record (EBR) software platform is engineered to meet these demands, automating Device History Records (DHR), batch record management, and ensuring rigorous data integrity and retention. Our solutions are designed to streamline operations, significantly reduce costs, and accelerate time-to-market, thereby enhancing your competitive edge.
Streamline Your Medical Device and Pharmaceutical Operations with Cutting-Edge EBR Technology
Elevate your operational efficiency, compliance, and market readiness with our state-of-the-art Electronic Batch Record (EBR) software. Designed specifically for the medical device and pharmaceutical industries, our platform automates critical processes like Device History Records (DHR) and batch management, ensuring seamless compliance with global standards such as 21 CFR Part 11 and GAMP 5. With customizable workflows and robust integration capabilities, our solution delivers a unified, paperless management system that transforms the way you manage data, compliance, and production
Enquire NowOur Customers











Tailored Solutions from Design to Support:
We offer end-to-end solutions tailored to the unique needs of the medical device and pharmaceutical industries. From the initial design specification to implementation, and ongoing system support and hosting, our approach is holistic. Our platform’s flexibility allows for the customization of data collection forms and process approval workflows, ensuring they align perfectly with your specific operational requirements.
Uncompromising Compliance and Integrity:
Adhering to the stringent standards of the industry, our software meets international compliance requirements, including 21 CFR Part 11 for electronic records and signatures, and GAMP 5 guidelines for Good Automated Manufacturing Practice. A comprehensive audit trail feature is embedded within our solutions, ensuring full traceability and accountability, which is critical in regulatory audits and quality control processes.
Seamless Integration for a Unified System:
Understanding the complexity of medical device and pharmaceutical operations, our software is designed to integrate smoothly with your existing ERP systems and accounting packages. This integration facilitates a cohesive, paperless business management ecosystem, enhancing operational visibility and decision-making.
What our trusted clients say
Related Products
All of our products and platforms are web based and run on a SQL back end database so they can scale to enterprise type requirements where necessary. Making our technology very adaptable and customizable.